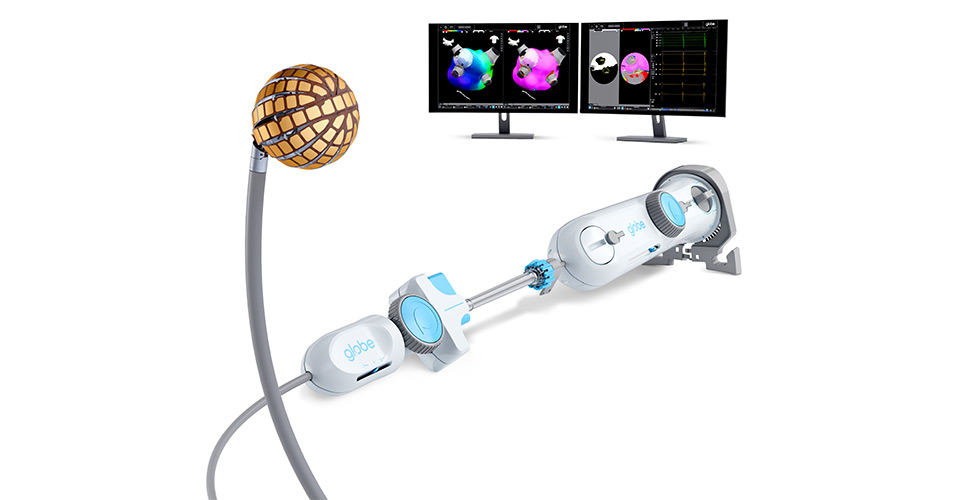
Vancouver-based Kardium has secured U.S. Food and Drug Administration approval for its Globe® Pulsed Field System, opening the American market to its atrial fibrillation treatment technology. The firm also received 510(k) clearance for its Globe Introducer sheath and mapping software.
The Globe System integrates high-density mapping and ablation, enabling both single-shot pulmonary vein isolation and targeted ablation through a single catheter. Results from the PULSAR study showed 78% freedom from arrhythmia at one year with no device-related safety events. The FDA approval follows Kardium’s US$250 million financing round, strengthening Canada’s position in the global medtech industry.
Want to know more? Check out the source code on Techcouver.